体外PK/PD模型(In vitro toxicology)
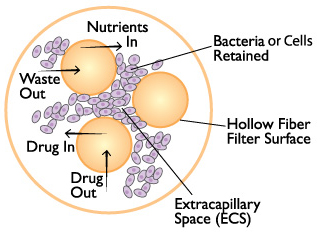
体外PK/PD模型是指借助体外装罝来模拟药物在体内的药代动力学过程和药效动力学的研究方法。与传统的抗菌药物药效学研究方法相比,该模型能够直接体现体内抗菌药物动态变化过程中与细菌或细胞的相互作用,且不受实验动物的限制。
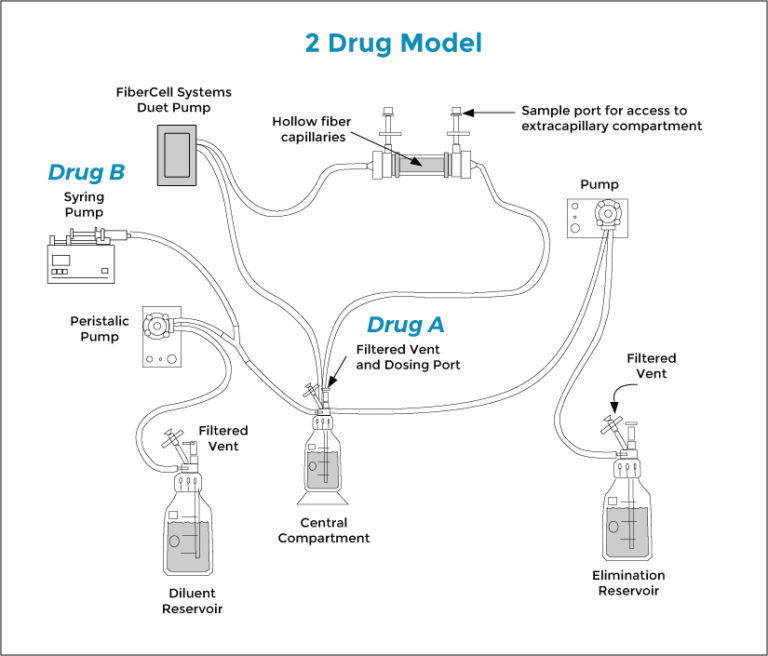
FiberCell系统可以实现单一药物或同时两种药物的体外PK/PD模型, 更精确的监控剂量和代谢状况。

欧盟药品监督管理局(EMA)已批准FiberCell系统用于体外毒性测试,得到的实验数据可用于临床申报。
参考文献
Comparison of in vitro static and dynamic assays to evaluate the efficacy of an antimicrobial drug combination against Staphylococcus aureus: Broussou, D. et al; PLOS ONE Jan 2019 [open access]
Clinical Regimens of Favipiravir Inhibit Zika Virus Replication in the Hollow-Fiber Infection Model:Camilly P. Pires de Mello et al.; Antimicrob Agents Chemother 2018 62 [abstract]
Differential Activity of the Combination of Vancomycin and Amikacin on Planktonic vs. Biofilm-Growing Staphylococcus aureus Bacteria in a Hollow Fiber Infection Model: Broussou, D. et al; Front. Microbiol., 27 March 2018 [open access]
Optimization and evaluation of piperacillin plus tobramycin combination dosage regimens against Pseudomonas aeruginosa for patients with altered pharmacokinetics via the hollow-fiber infection model and mechanism-based modelling: Yadav, R. et al; AMS 2018 (62) 3 [abstract]
Evaluation of Activity and Emergence of Resistance of Polymyxin B and ZTI-01 (Fosfomycin for Injection) against KPC-Producing Klebsiella pneumoniae: Diep, J.K. et al; Antimicrob. Agents Chemother. 2018 (62) 2 [open access]
Oseltamivir-zanamivir combination therapy suppresses drug-resistant H1N1 influenza A viruses in the hollow fiber infection model (HFIM) system: Pires de Mello, C.P.; European Journal of Pharmaceutical Sciences 111 (2018) 443-449 [open access]
Polymyxin Combinations Combat Escherichia coli Harboring mcr-1 and blaNDM-5: Preparation for a Postantibiotic Era. Bulman Z.PP; mBio 8(4)2017 [open access]
In vitro pharmacodynamic evaluation of ceftolozane/tazobactam against β-lactamase-producing Escherichia coli in a hollow-fibre infection model Soon, R et al. International Journal of Antimicrobial Agents 2017 49(1)[open access]
From lead optimization to NDA approval for a new antimicrobial: Use of pre-clinical effect models and pharmacokinetic/pharmacodynamic mathematical modeling. Drusano G.L.; Bioorg Med Chem. 2016[abstract]
Linezolid for Infants and Toddlers With Disseminated Tuberculosis: First Steps: Deshpande, D. et al.; Clin Infect Dis. 2016, 63 (3)S80-87 [abstract]
Concentration-Dependent Synergy and Antagonism of Linezolid and Moxifloxacin in the Treatment of Childhood Tuberculosis: The Dynamic Duo: Deshpande, D. et al.; Clin Infect Dis. 2016, 63 (3): S88-S94.[open access]
A Faropenem, Linezolid, and Moxifloxacin Regimen for Both Drug-Susceptible and Multidrug-Resistant Tuberculosis in Children: FLAME Path on the Milky Way: Deshpande, D. et al.; Clin Infect Dis. 2016, 63(3): S95-S101.: S88-S94.[open access]
Thioridazine as Chemotherapy for Mycobacterium avium Complex Diseases: Deshpande, D. et al.; Antimicrob. Agents Chemother. 2016 60 (8) 4652-4658 [open access]
Azithromycin Pharmacodynamics Against Non-Typeable H. Influenzae: Fisher, J., Yale University Public Health Theses 2016 [open access]
EMA Qualification opinion: In-vitro hollow fiber system model of tuberculosis (HSF-TB):EMA/CHMP/SAWP/47290/2015
Continuous culture of Cryptosporidium parvum using hollow fiber technology: Morada, M. et al.; Int J Parasitol. 2016 Jan;46(1):21-9 [related presentation PDF]
A long-term Co-perfused Disseminated Tuberculosis-3D Liver Hollow Fiber Model for Both Drug Efficacy and Hepatotoxicity in Babies: Srivastava, S. et al.; EBioMedicine 2016; 6:126-138 [open access]
Preclinical Evaluations To Identify Optimal Linezolid Regimens for Tuberculosis Therapy: Brown, A. et al.; mBio vol. 6 (6) [open access]
Strategic Regulatory Evaluation and Endorsement of the Hollow Fiber Tuberculosis System as a Novel Drug Development Tool: Romero, K., Clay, R. and Hanna, D.; Clinical Infectious Diseases 2015 61 (1): S5–9 [open access]
In Vitro Pharmacodynamics of Various Antibiotics in Combination against Extensively Drug-Resistant Klebsiella pneumoniae Lim, Tze-Peng et al.; Antimicrobial Agents and Chemother. 2015 59(5): 2515–2524.[open access]
Correlations Between the Hollow Fiber Model of Tuberculosis and Therapeutic Events in Tuberculosis Patients: Learn and Confirm: Gumbo, T.;2015 [open access]
The Hollow Fiber Infection Model: Principles and Practice: Cadwell, J.;J Adv Antibiotics and Antibodies 2015, 1(1) [open access]
Colistin and doripenem combinations against Pseudomonas aeruginosa: profiling the time course of synergistic killing and prevention of resistance:Ly,N.S. et al.; Journal of Antimicrobial Therapy 2015 70(5): 1434-1442 [open access]
Hollow Fiber System Model for Tuberculosis: The European Medicines Agency Experience: Cavaleri, M. and Manolis, E.; Clin Infect Dis. (2015) 61 (suppl 1): S1-S4.[open access]
The in vitro hollow fiber system model has been qualified by the European Medicines Agency as a methodology for use in support of selection and development of antituberculosis regimens. More data are expected to be generated in the future to further characterize its value.
Rapid Drug Tolerance and Dramatic Sterilizing Effect of Moxifloxacin Monotherapy in a Novel Hollow-Fiber Model of Intracellular Mycobacterium kansasii Disease: Srivastava, S. et al.; Antimicrob. Agents Chemother. 2015, 59(4) [open access]
Model System to Define Pharmacokinetic Requirements for Antimalarial Drug Efficacy: Bakshi R., Shapiro A. et al.; Science Translational Medicine 2013:Vol. 5, Issue 205 [open access]
Thioridazine Pharmacokinetic-Pharmacodynamic Parameters “Wobble” during Treatment of Tuberculosis: a Theoretical Basis for Shorter-Duration Curative Monotherapy with Congeners Musuka, S. et al.; Antimicrob. Agents Chemother. 2013 vol. 57 no. 12 5870-5877 [open access]
PK/PD models in antibacterial development: Velkov, T. et al.; Curr Opin Microbiol . 2013, 16(5) [open access]
Hollow-fiber pharmacodynamics studies and mathematical modeling to predict the efficacy of amoxicillin for anthrax postexposure prophylaxis in pregnant women and children. Louie, A et al.; Antimicrob Agents Chemother 2013; 57:5946–60. [open access]
Relationship between Ceftolozane-Tazobactam Exposure and Drug Resistance Amplification in a Hollow-Fiber Infection Model: VanScoy, B. et al.; Antimicrobial Agents and Chemotherapy 2013 p 4134 – 4138. [open access]
The Hollow Fiber Infection Model for Antimicrobial Pharmacodynamics and Pharmacokinetics:Cadwell, J.; Adv Pharmacoepidem Drug Safety 2012 [open access]
In Vitro Activity of MK-7655, a Novel Beta-Lactamase Inhibitor, in Combination With Imipenem Against Carbapenem-Resistant Gram-Negative Bacteria: Hirsch,E.B. et al.; Antimicrobial Agents and Chemotherapy 2012; 56(7): 3753–57 [open access]
Simulated Antibiotic Exposures in an In Vitro Hollow-Fiber Infection Model Influence Toxin Gene Expression and Production in Community-Associated Methicillin-Resistant Staphylococcus aureus Strain MW2: Pichereau, S. et al.; Antimicrob Agents Chemother. 2012 Jan; 56(1): 140–147. [open access]
Effect of Half-Life on the Pharmacodynamic Index of Zanamivir against Influenza Virus Delineated by a Mathematical Model: Brown, A. et al.; Antimicrob. Agents Chemother. 2011 55 (4) 1747-1753[open access]
Antiviral pharmacodynamics in hollow fibre Bioreactors: McSharry, J. et al.; Antiviral Chemistry & Chemotherapy 2011; 21:183–192 [open access]
Pharmacokinetic Mismatch Does Not Lead to Emergence of Isoniazid- or Rifampin-Resistant Mycobacterium tuberculosis but to Better Antimicrobial Effect: a New Paradigm for Antituberculosis Drug Scheduling: Srivastava, S. et al; Antimicrob. Agents Chemother. 2011 55 (11): 5085-5089 [open access]
Moxifloxacin Pharmacokinetics/Pharmacodynamics and Optimal Dose and Susceptibility Breakpoint Identification for Treatment of Disseminated Mycobacterium avium Infection :Deshpande,D. et al.;Antimicrob Agents Chemother. 2010 54(6): 2534–2539. [open access]
Optimizing the Culture of Plasmodium Falciparum in Hollow Fiber Bioreactors :Preechapornkul,P. et al.;Southeast Asian J Trop Med Public Health. 2010 41(4): 761–769 [open access]
Ethambutol Optimal Clinical Dose and Susceptibility Breakpoint Identification by Use of a Novel Pharmacokinetic-Pharmacodynamic Model of Disseminated Intracellular Mycobacterium avium:<>Deshpande, D. et al.; Antimicrob Agents Chemother. 2010 54(5): 1728–1733 [open access]
Prediction of the Pharmacodynamically Linked Variable of Oseltamivir Carboxylate for Influenza A Virus Using an In Vitro Hollow-Fiber Infection Model System. McSharry, J.; Antimicrob. Agents Chemother. 2009 53 (6) 2375-2381 open access]
Pharmacodynamics of Cidofovir for Vaccinia Virus Infection in an In Vitro Hollow-Fiber Infection Model System. McSharry, J.; Antimicrob. Agents Chemother. 2009 53(1) 129-135 [open access] – system diagram
Pharmacodynamic Characterization of gemcitabine cytotoxicity in an in vitro cell culture bioreactor system. Kirstein MN, Brundage RC, Moore MM, Williams BW, Hillman LA, et al. 2008 Cancer Chemother Pharmacol 61: 291-299.
Comparative Pharmacodynamics of Gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa: Tam, V. et al; Antimicrob Agents Chemother. 2006 50(8): 2626–2631
Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. Gumbo, T. et al.; J Infect Dis. 2004 Nov 1;190(9):1642-51. [open access]
Copy right 2012 by Shanghai XP Biomed Ltd. All Rights Reserved
沪 ICP备12039761号-1 沪ICP备12039761号-2 沪ICP备12039761号-3 沪ICP备13002267号-1